Phase Transformations in the Absence of an Externally Applied Stress
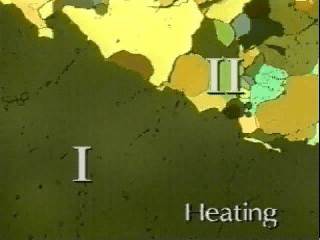
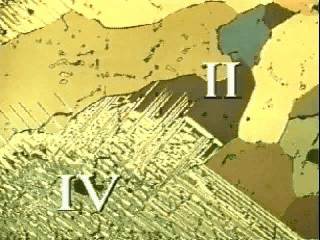
Form IV (distorted CsCl structure) and form III (NiAs structure type?) are both orthorhombic and the transformation between these forms are reconstructive. Proton magnetic resonance (PMR) studies indicate that, at room temperature and above, the NH4 ions are undergoing random reorientations, while X-ray studies indicate that the NO3 ions are in fixed orientations only in forms IV and III. On heating III transforms to II which is tetragonal and structurally similar to IV: consequently the III to II transformation is also reconstructive.
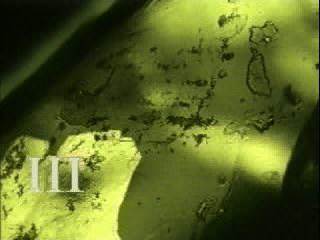 |
Fig. 4 Single crystal of Phase IV transforms on heating at about 32°C into phase III. A 'faceted' interface advances slowly but steadily from the bottom left corner. |
 |
Fig. 5 Transformation to III is now complete. Rotation of the specimen in the movie (crossed polarisers) shows it is an extremely fine-grained polycrystal.. |
 |
Fig. 6 III transforms on heating at ~85°C to II. The movie shows an interface which has a complex shape and which advances from south to north. |
In form II the NO3 ions are undergoing rotational disorder in their plane and electrical conductivity and PMR studies indicate that the NH4 ions are diffusing relatively freely compared with forms IV and III. However, the II to I transformation is displacive and the rotational disorder of the NO3 ions in form I is no longer restricted to the plane of the ion. Thus both ions in I are essentially spherically symmetric and, consequently, I is cubic with the CsC1 structure. PMR evidence indicated a further, very significant, increase in the diffusion of the NH4 ions at the II to I transformation, so that form I behaves like a superionic conductor. Forms II and IV are simply slight distortions of form I, brought about essentially by increasing rotational and/or translational order on cooling. The reconstructive transformations into III from either II (on cooling) or IV (on heating) occur only if some solvent for NH4NO3 is present, even in very small amounts. In perfectly dry specimens displacive transformations between IV, II and I only occur.
Although NH4NO3 is not a structural analogue of any important rock-forming mineral, it is, however, a very suitable material for studying the microstructural changes that take place during different types of solid-state phase transformations. The microstructural changes have been observed in thin films in the light microscope. Being reconstructive, the transformations involving form III usually lead to a significant reduction in grain size (Fig 5). However, the "massive" transformations between II and I lead to significant grain growth. The II to IV transformation is "martensitic" – small lenses of IV propagate rapidly across large grains of II, and then thicken steadily until all of the host grain is consumed (Fig 9).