Experiments
The experimental magmatic system consists of three crystalline phases and melt (Figure 1a). The crystalline phases are ammonium thiocyanate - NH4SCN (W in Figure 1a, white phase hereafter), ammonium chloride - NH4Cl (C in Figure 1a, cube phase hereafter), and diammonia tetrathiocyanato cobaltate - (NH4)2[Co(SCN)4]nH2O (B in Figure 1a, blue phase hereafter). The liquid phase (M in Figure 1a) is a hydrous solution with dissolved ions of NH4+, Cl-, SCN- and [Co(SCN)4]2-. The physical, optical and petrographic properties are given in Figure 1b and details about the procedures of sample preparation are described in Means and Park (1994).
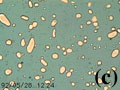
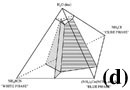
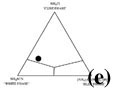
Figure 1c shows the crystallization sequence of the bulk composition used in this study. The first-crystallizing phase (thus liquidus phase) is the cube phase, although initial crystallization from the crystal-free melt is not shown. Then, white phase crystals nucleate and grow, and finally blue phase crystals appear. The color of melt becomes pale as blue crystals appear at the end of Figure 1c, suggesting the incorporation of the blue [Co(SCN)4]2- ions of the melt into blue phase crystals. Based on the observation of the appearance and disappearance of the crystals during cooling and melting experiments, and the observation that the crystalline phases do not react with melt and produce a new crystalline phase, a quaternary eutectic phase diagram (Figure 1d) is constructed for the experimental system. The bulk composition of the experimental system is shown on the section parallel to the plane of the cube-white-blue phase (Figure 1e).
Experimental Apparatus
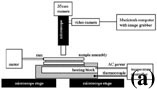
The experimental apparatus used in this study is shown in Figure 2a. A mini-press designed by Janos Urai is attached on the stage of a petrographic microscope (Figure 2b and c) and a 35mm camera and a video camera attached to a Macintosh through a frame grabber are mounted on a microscope for recording the experimental images. Temperature conditions during experiments were set up by a programmable temperature controller (Eurothem model 808) for different types of the thermal histories (e.g. constant temperature, cooling and cycling). A Macintosh utility, QuicKeys, was used when recording the zero strain-rate experiments while unattended.
The experimental samples were prepared using a sets of glass slides on which scratches were made using a glass-cutting pen. Thicker crystals grow from the melt into the scratched area of the glass slides and they serve as grips to cause deformation in the area between scratches (Figure 3a). The scratch geometries used for deformation experiments are pure shearing (Figure 3b), simple shearing (Figure 3c) and general (or mixed) shearing.