Microstructural Development at Zero Strain Rate
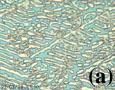
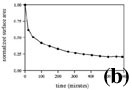
Dendrite segmentation is a process by which primary crystals of dendritic form in melt become subdivided into pieces surrounded by melt (Kattamis et al, 1967; Kahlweit, 1968). Dendrite segmentation can therefore be a type of ‘nucleation’ process. An example is shown in Figure 4a. Conventional interpretation of the last frame in the time-lapse movie of Figure 4a would be that each crystal in the last frame represents one nucleus and larger crystals had a longer growth time or a faster growth rate. However, as shown in the sequence of the time-lapse movie, crystals in the last frame of Figure 4a are not crystals formed by accretion around individual nuclei, but crystals which survived during segmentation of a dendritic crystal and coarsening. The first frame in Figure 4a was taken moments after crystallization of a white phase dendritic crystal. Since a dendritic crystal has a large surface area to volume ratio, it is not stable due to the excessive surface energy and spontaneously breaks down by segmentation. Segmentation of a dendrite, indicated by separated pieces of arms, has already begun at the stage of the first frame. After segmentation, coarsening (or dissolution of smaller crystals and growth of larger crystals) occurs and results in very different texture at the end. Measurements of the interfacial surface over time are shown in Figure 4b. A remarkable decrease in the surface area (in fact, perimeter of crystals) during the first 30 minutes can be seen. The rate of decrease in surface area becomes slower over time, since the driving force, the interfacial surface energy, decreases over time. There is also an apparent volume decrease of crystalline phases during segmentation and coarsening. This is because the initial dendritic crystals do not fully occupy the space between two glass slides while the crystals fully occupy the space after segmentation and coarsening.
Dendritic crystallization is common in cast metals (Rostoker & Dvorak, 1977) and has been found in rapidly cooled experimental silicate systems (Lofgren, 1980). Dendritic crystals also occur in chilled margins of plutons (Shannon et al, 1982; Tegner et al, 1993). Segmentation of dendrites and subsequent coarsening has been known in metallurgy (Kattamis et al, 1967). If segmentation and coarsening of dendrite is possible in silicate systems, this mechanism provides an additional nucleation mechanism, augmenting the conventional nucleation mechanism in which the nucleation rate is the number of nuclei forming from melt per volume per time. If segmentation and coarsening lead to formation of ‘nuclei’ or growth centers around which crystals will grow, the ‘nucleation rate’ will be, in fact, the sum of a conventional nucleation rate and the rate of growth center formation by dendrite segmentation. This may introduce complications when inferring nucleation rate and growth rate using crystal size distribution (e.g. Cashman & Marsh, 1988).
Grain- and Phase-Boundary Migration
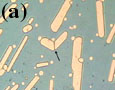
Grain-boundary migration is a process by which a grain boundary between two grains of the same phase migrates to lower the surface free energy or strain energy. Grain-boundary migration is commonly observed in our samples. Figure 5a shows an example of grain-boundary migration between two growing white phase crystals during cooling, resulting in an unexpected change of grain-boundary geometry. The growth-impingement grain boundary (indicated with an arrow) formed between the two white phase crystals in the first frame of Figure 5a starts to migrate and the migrated grain boundary becomes rational with respect to the left white phase crystal in the frames near the end of the time-lapse movie. The order of crystallization, judged from the last frame of Figure 5a, would be such that the left white phase crystal occupied the growth space first, stopped growth and the right white phase crystal formed a growth-impingement boundary. However, the recorded sequence clearly shows the boundary is not a simple impingement boundary but a migrated boundary which has changed its orientation and position. Grain-boundary migration in this example is likely to be driven by surface energy (grain-boundary energy), since strain energy in the lattice is negligibly small because of no concurrent deformation.
Phase-boundary migration is a process by which a phase boundary between two phases migrates by chemical reaction or structural transformation. Phase-boundary migration also occurs in this system although the rate is much slower than grain-boundary migration. Figure 5b shows migration of the phase boundary between a blue phase crystal and a white phase crystal, with abundant melt nearby. The blue phase crystal has a square form because its tetragonal c-axis is perpendicular to the plane of the photomicrograph.
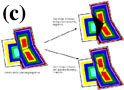
During phase-boundary migration between two chemically different phases, transfer of atoms which are involved during chemical reaction is always necessary, unlike grain-boundary migration in which simple detachment and attachment of atoms from one grain to another is necessary. If diffusion along the boundary region is faster than diffusion through the crystals, the boundary region can be viewed as the channel that is connected to a main reservoir of melt during melt-present phase-boundary migration. If diffusion along the boundary is fast or long-range diffusion occurs, the part of a crystal which grows by phase-boundary migration will have a composition in equilibrium with the main melt. However, when only short-range diffusion occurs, the ‘melt’ phase in the boundary region can have a chemistry slightly different from that of the main melt, and the part of a crystal growing by phase-boundary migration will have an equilibrium composition with the local boundary region ‘melt’. Different mineral zonation patterns will develop depending on the range of diffusion (Figure 5c). Such zonation patterns may be useful when identifying the phase-boundary migration from igneous rocks.
During grain-boundary migration, although melt-connectivity is not required, the grain-boundary region can be also filled with the ‘melt’ of bulk melt composition or local melt composition, depending on the range of diffusion. Similar mineral zonation patterns as in Figure 5c are expected after grain-boundary migration.
Grain Migration through Melt
Grain migration, in polycrystalline aggregates, is accompanied by grain-boundary migration in one direction at the opposite sides of a grain (Fig. 4, Urai et al, 1986). The atoms or material points in a grain where grain migration occurs, however, are fixed in position with respect to the material points outside the grain. Thus, the process of grain migration is markedly different from grain-boundary sliding which requires movement of material points within a grain. A similar process is observed in the experimental magmatic system. The white phase crystals indicated with arrows in Figure 6 migrate by growth at one end and simultaneous dissolution at the other end of the same grain. Dissolution and growth of the white phase crystals can be explained by the local concentration gradient existing between the crystals (migrating and neighboring crystals). The concentration gradient between the crystals is established since the solubility of the crystals (or the equilibrium concentration of the crystals) is dependent on the size of crystals (Gibbs-Thomson equation, Shewmon, 1965).
Migration of crystals by grain migration in melt is completely different from the well-known mechanisms such as convection (material transport with medium). The material in crystals that changed their position by grain migration can be much younger than their original nuclei. Zonation patterns in crystals may record grain migration.