 CET/UWA Microstructure Course
CET/UWA Microstructure Course CET/UWA Microstructure Course
CET/UWA Microstructure CourseGEOS 5505 Microstructure Prac Day 1
In this prac we will look at various numerical simulations and analogue experiments that demonstrate how single micro-scale processes behave, and how they interact.
1) Molecular dynamics simulations of the states of matter. Starting with the simplest possible multi-atom system, we can examine a whole range of behaviours as a function of temperature using an online 2D molecular dynamics simulation called atomdemo:
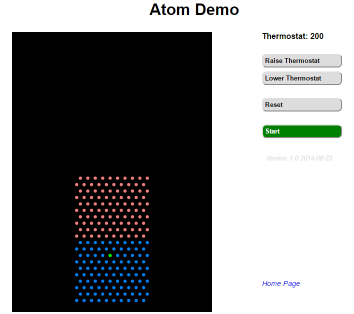
http://xmd.sourceforge.net/other/atomdemo/atomdemo.html
ATOMDEMO is a "lite" version of molecular dynamics simulation, which
is used to study atoms and molecules the fields of chemistry, drug design,
physics and materials. The program works along simple lines - it calculates the
forces on the atoms, moves the atoms forward a short period of time, recalculates
the forces, and so on. The challenge of this technique is reproducing the
forces between atoms - ATOMDEMO uses a simple pair potential model (called a Lennard-Jones Potential" that was
designed for efficient computation. V is the potential between atoms, r is the atom spacing, sigma and e are constants which differ for each type of atom, but are constant during the expirements here
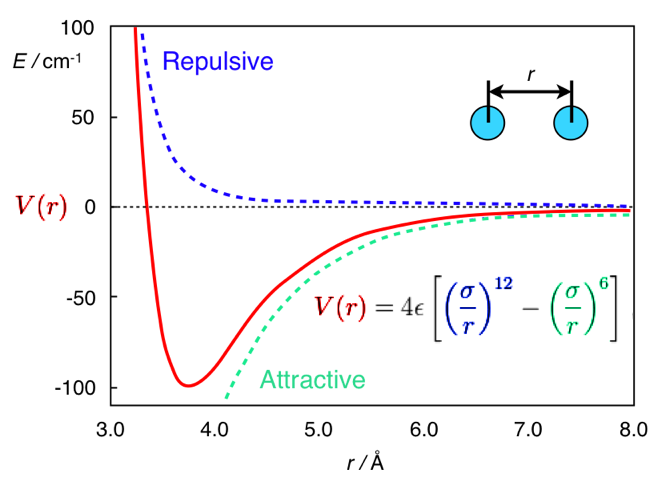
Unlike most molecular dynamics software, ATOMDEMO includes the force of gravity. This makes it easier to compare the simulations to everyday experience. Also unlike most molecular dynamics software, ATOMDEMO operates in two dimensions only. This simplifies display and comprehension.
There are only 4 controls for this simulation, two buttons that Raise or Lower the current ambient temperature by 50 degrees (Kelvin), a button to Reset the simulation to 200 degrees and the starting atom configuration; and finally a button to start the Start or Pause the simulation.
The atoms come in 3 colours, but they all obey the same atomistic interactions, the coulours are just to make it easier to follow the behaviour.
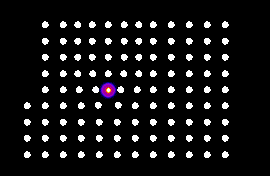
The starting configuration shows us a set of atoms arranged as a crystal lattice. Question 01. Before you run the experiment, what pattern do the atoms make? What happens when you run the experiment the first time ((Click on the Start button, let the expirement run for a while and then click on Pause)? What pattern do the atoms make now? Has the overall shape of the crystal changed? Are the pink and blue atoms mixed? Are these atoms behaving as a crystal, a liquid or a gas?
Question 02. Reset the experiment, Raise the temperature to 600, then start the experiment, now what happens? After a while, Pause the experiment? What pattern do the atoms make? Are the pink and blue atoms mixed? Do the atoms fill the whole box? Are these atoms behaving as a crystal, a liquid or a gas?
Question 03. Now Lower the temperature to 0, Continue the experiment and what happens? What pattern do the atoms make? Are these atoms behaving as a crystal, a liquid or a gas?
Question 04. Reset the experiment, Raise the temperature to 1200, then start the experiment, now what happens? What pattern do the atoms make? Are the pink and blue atoms mixed? Do the atoms fill the whole box? Are these atoms behaving as a crystal, a liquid or a gas? Now Pause the experiment, drop the Temperature to 0, and Continue the experiment. What real-world meteorological phenomenom are we simulating?
In order to make a 1D phase diagram, you will need to systematically run the expriemnts at a range of temperatures.Question 05. Estimate the freezing point of this material? Estimate it's boiling point? As you may know gravity, an external force, affects the temperatures at which materials undergo phase changes.Now load the gravity-free version of this experiment. Estimate the freezing point of this material under zero gravity? Estimate it's boiling point under zero gravity? You can now start to make a simple 2D (Gravity vs temperature) phase diagram. The atoms under zero gravity mostly stay as a compact blob, why is this?
2) Molecular dynamics simulations of crystallography and deformation. Now we will examine a system with two different types of atom, with and without a charge for each type of atom:
http://mw.concord.org/nextgen/#interactives
Now go to the Physics section, then Intermolecular Attractions, then Charged and Neutral Atoms
a) Charged and Neutral Atoms. There are two kinds of attractive forces shown in this model: Coulomb forces (the attraction between ions) and Van der Waals forces (an additional attractive force between all atoms). Question 06:What kinds of patterns tend to form with charged and neutral atoms? How does changing the Van der Waals attraction or charging the atoms affect the melting and boiling point of the substance?
b)
Now go to the Mechanical Properties section, then compare the
deformation of materials with Ceramic and Metal Forces. This model shows a
ceramic (such as pottery, brick or mineral), an ionic lattice. In this model
you can use a downward force to bend a small ceramic beam. Use different
amounts of force to bend the beam. Question 07:What happens to the
beam when you use a small force or a large force? What happens when you release
the force? Compare the structure and behaviour of this beam to the metal beam.
How do their behaviours differ and why?
c)
With each example, and Large Force selected for
the Ceramic case and Medium Force for the Metallic example, run the simulation
until it JUST starts to fail. You can use the double
arrows to run back the experiment and observe how each material deforms.Question 08: Does it
fail all at once, or can you see progressive failure? What types of behviour do these movies represent?
3) Now we are going to go upscale
 3D
3D 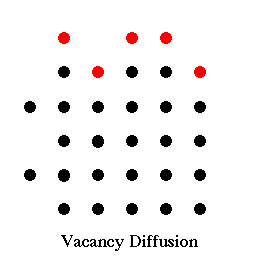
D) Edge Dislocations Motion. In these movies a single horizontal glide plane is activated by elastic strain build up in a crystal. Watch as the lattice bonds in the glide plane get stretched, and then switch one by one to a new orientation, and then once the dislocation has passed through the crystal, how this allows the further slip of the crystal on the glide plane.
5) Mechanical experiments in analogue materials.
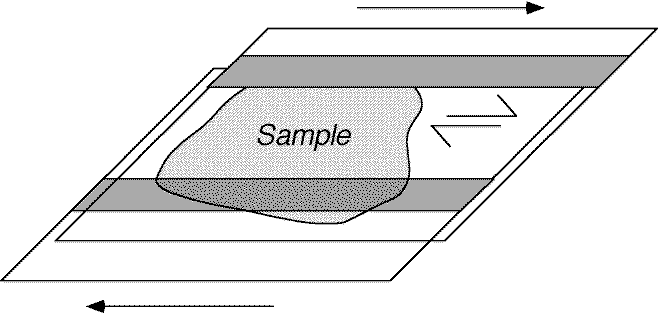
Real rocks are generally very hard to deform at room T & P, however by examining the behaviour of non-geological materials we can learn a lot about the mechanics of deformation and microstructure formation. We will look at four small excerpts from some experiments performed by Youngdo Park, Win Means, and Jin-Han Ree from the State University of New York at Albany, using an organic chemical called Octachloropropane (C3Cl8). All the experiments were performed by shearing the material between two glass plates, with thin frosted strips acting as grips as shown on the next figure...
A) Movie 01- Fracture and flow of polycrystalline OCP Fracturing and frictional sliding are often neglected deformation mechanisms in rocks that also show crystalline plasticity.

Question 15:Estimate the shear strain shown in this movie, and guesstimate the amount of shearing taken up by the sliding plane about 20% up the image.

B) Movie 02 Plasticity and fracture in polycrystalline OCP
These two movies show fracturing and sliding behaviour in OCP at the same time as twinning, and at the same time as intra-crystalline plasticity. The final frame of the movie shows the microstructure in plane light, to highlight the fractures. Describe the deformation mechanisms evident in the two movies, and explain how they allow shape change in the aggregate.
C)Movie 03 Undulose extinction in polycrystalline OCP Undulose Extinction
Play the movie below. In this movie we can see the development of undulose extinction in two grains A & B. Notice the contrast in behaviour between the two grains, with one showing very sharp variations within the grain in orientation (and hence extinction), and the other showing only gradual changes. What deformation mechanisms can you see evidence for in this movie.
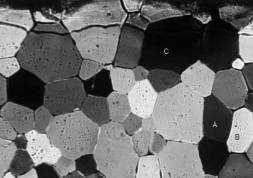
6) Evidence of crystalline plasticity in naturally deformed
rocks.
a) Quartzite Weak to moderate deformation of a quartzite with minor micas at low metamorphic grade. Original clastic quartz grains show numerous features of intra-crystalline plasticity, including deformation bands, and undulose extinction.

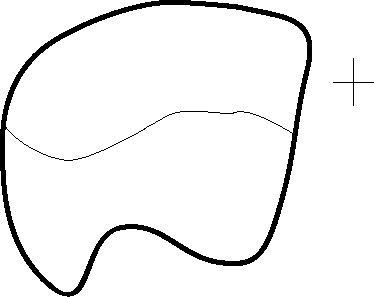
Question 19:Now look at grain A in the top right hand corner, and make a sketch of its basal plane (assuming the dark bands are sub-perpendicular to the basal plane, as they usually are in low temperature deformed quartz).
b) Marble A typical low grade deformed
marble, showing extensive twinning.

c) Mt Kosciusko Granite

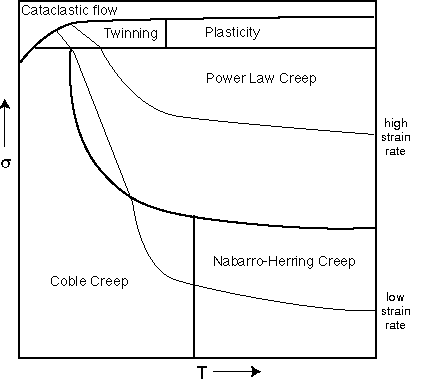
Question 22:Place a cross on the generic deformation mechanism
map below for both the quartz and the feldspar. On the same diagram show
the calcite from the previous picture as an arrow showing its history.
Copyright Mark Jessell & Paul Bons 2019